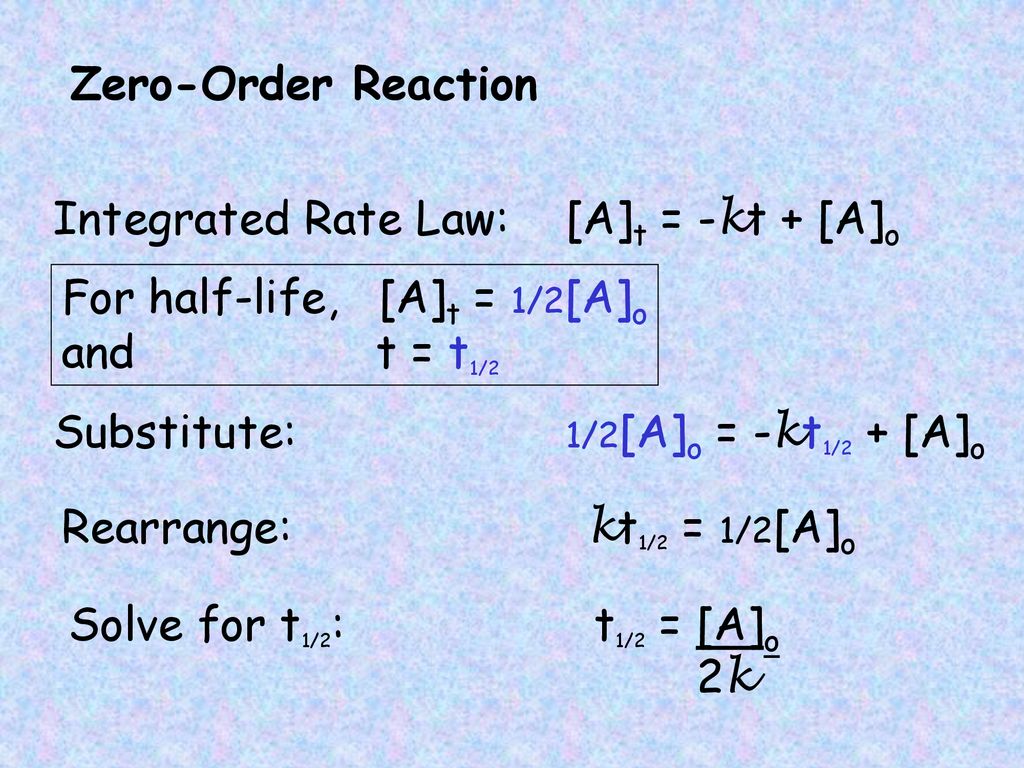
half life formula for zero order reaction
T ½ A o 2k For a first order reaction A products rate k A. A We can calculate the half-life of the reaction using Equation 453.

Derive An Expression To Calculate Time Required For Completion Of Zero Order Reaction
The half-life of a Zero-th order reaction is t A0 2kHere I derive this from the Integrated Rate LawAsk me questions.

. Which is the required equation for the half-life of zero order reactions. Equations for Half Lives For a zero order reaction A products rate k. The half-life formula used to calculate zero order reaction is t₁₂ A₀2k.
Remember the half-life of a reaction changes with the order of the reaction. If you found any kind of mistake in video or you have any sugge. The half-life of a second-order reaction is.
The equation given above shows that the half-life is dependent on the rate constant and the. Half life formula for nth order reaction A zero order reaction implies that the rate of the reaction does not depend on the concentration of the reactant. We know that the half-life of a zero order reaction is given by.
The units of k for a zero-order reaction are Ms the units of k for a first-order. Thanks for watching video if its really help you then make sure to share with your friends. The unit of half-life equation for zero order reaction is second 2.
The mathematical expression that can be employed to determine the half-life for a zero-order reaction is t12 R 02k For the first-order reaction the half-life is defined as t12 0693k. Rate kA0 k A zero-order reaction thus exhibits a constant reaction rate regardless of the concentration of its. The half-life of the reaction is denoted by t 12 and is expressed in seconds.
As for other reaction orders an equation for zero-order half-life may be derived from the integrated rate law. Half-Life versus Initial Concentration Graph. What are the units for first order.
As for all reaction orders the half-life for a zero-order. Half life Formula. The half-life of a zero-order reaction the formula is given as t 12 R02k The half-life of a first-order reaction is given as t 12 0693k.
Welcome to allThis class i shared to chemical kinetics unit rate constant derivated for zero order reaction and half life of zero order reactionzeroorder. T 1 2 0693 k 0693 15 10 3 min 1 46 10 2 min Thus it takes almost 8 h for half of the cis. The half-life of the reaction is clearly dependent on the rate constant as well as the initial concentration of the reactant as shown in the preceding equation.
Term half-lifeThe time required for a quantity to fall to half its value as measured at. It is to be noted that the formula for the half-life of a reaction varies with the order of the reaction. For zero-order reactions the differential rate law is.
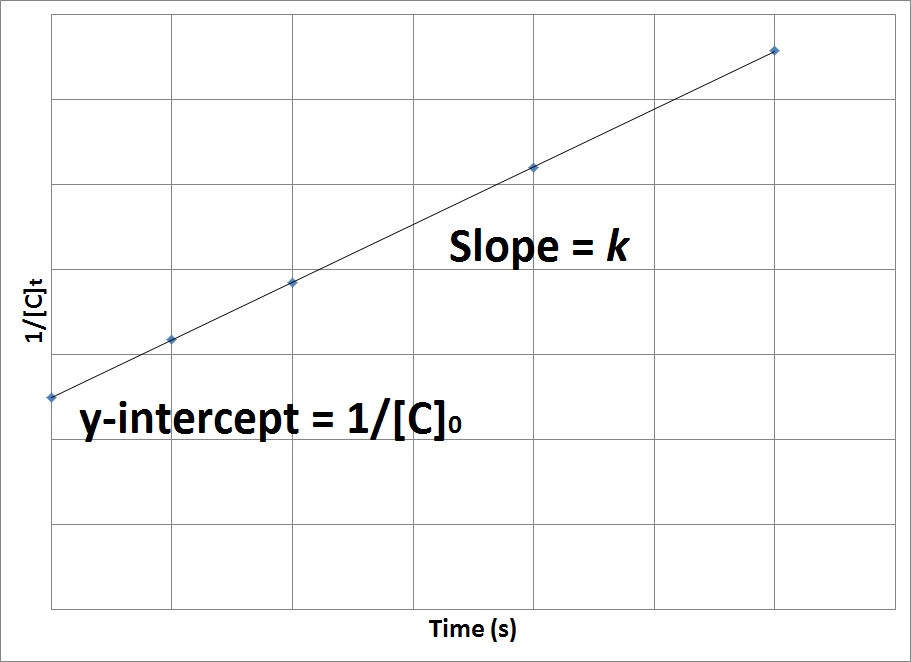
Which is again in the form of y mxc where y here will be equal. It is denote by t 12. The half-life of a zero-order reaction the formula is given as t 12 R 02 k The half-life of a first-order reaction is given as t 12 0693k The half-life of a second-order reaction is.
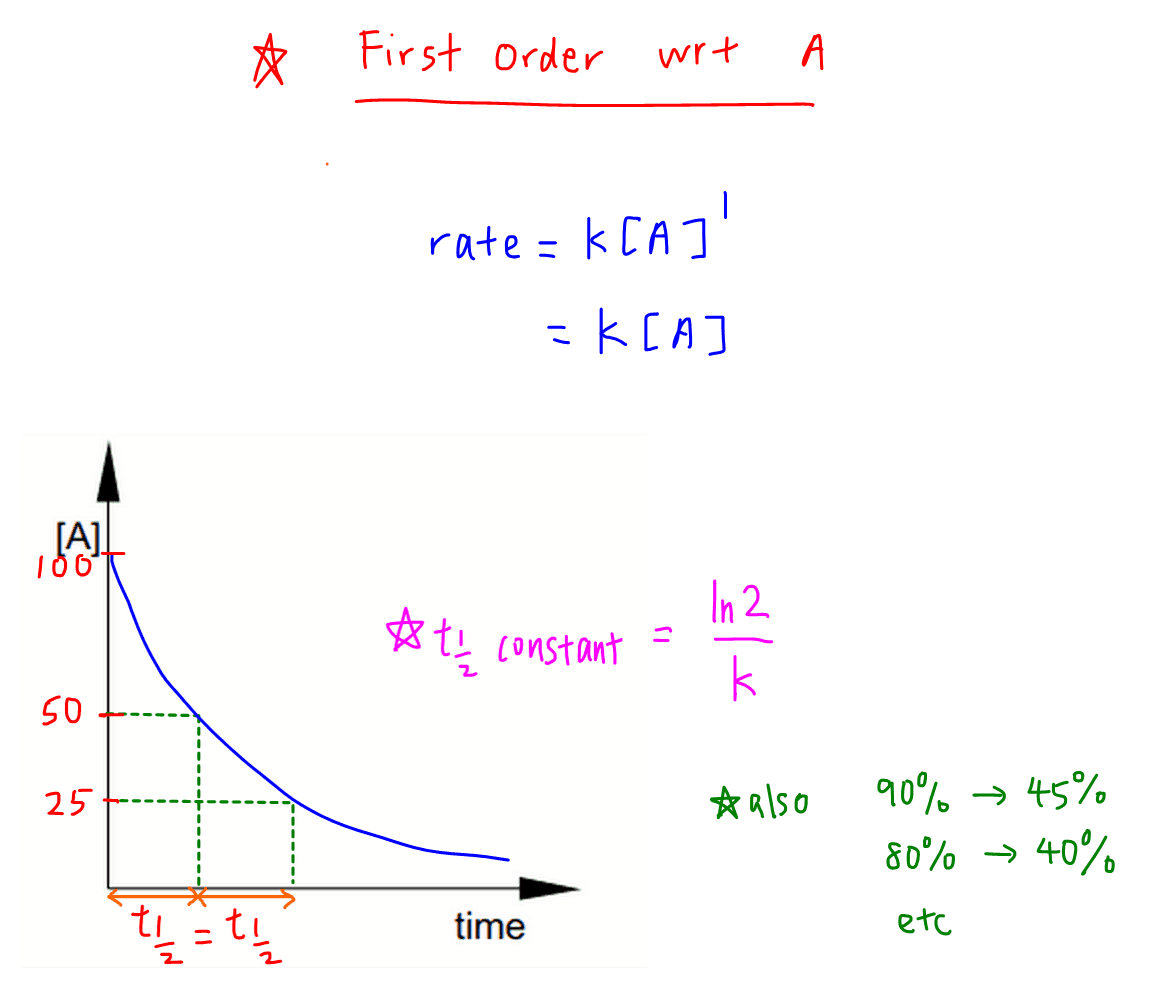
The half-life equation for a zero-order reaction is t_ frac 1 2frac A_ 0 2k t21 2kA0. T ½ 0693 k For a second order reaction 2A. Half-life formula and unit for.
Thus the half-life of a first-order reaction is given by 0693k. The time required to reduce initial concentration of the reactant to half of its initial value is called half life time or half life period. From the above-integrated equation we have.
A A0 - kt Now replacing t with half. The half-life for the first. T 1 2 A 0 2 k.
For a general reaction.
Concentration Time Relationships Integrated Rate Laws Introductory Chemistry

Determine The Half Life Of A Zero Order Reaction Youtube
Rate Equation And Order Of Reaction

Solved 3 The Reaction A B C Is Zero Order In A If The Chegg Com
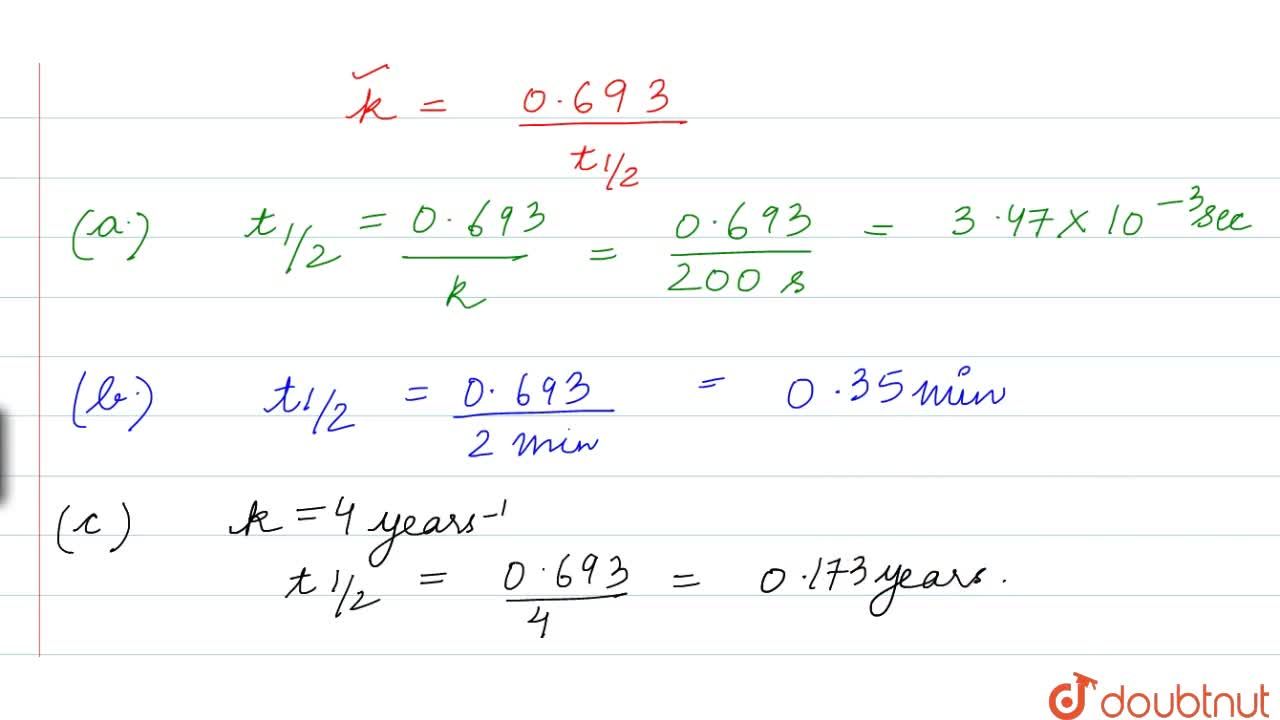
Calculate The Half Life Of A First Order Reaction From Their Rate Constants Given Below A 200s 1 B 2 Mi N 1 C 4years 1
Concentration Time Relationships Integrated Rate Laws Introductory Chemistry 1st Canadian Edition

Half Life Expressions Chemistnate
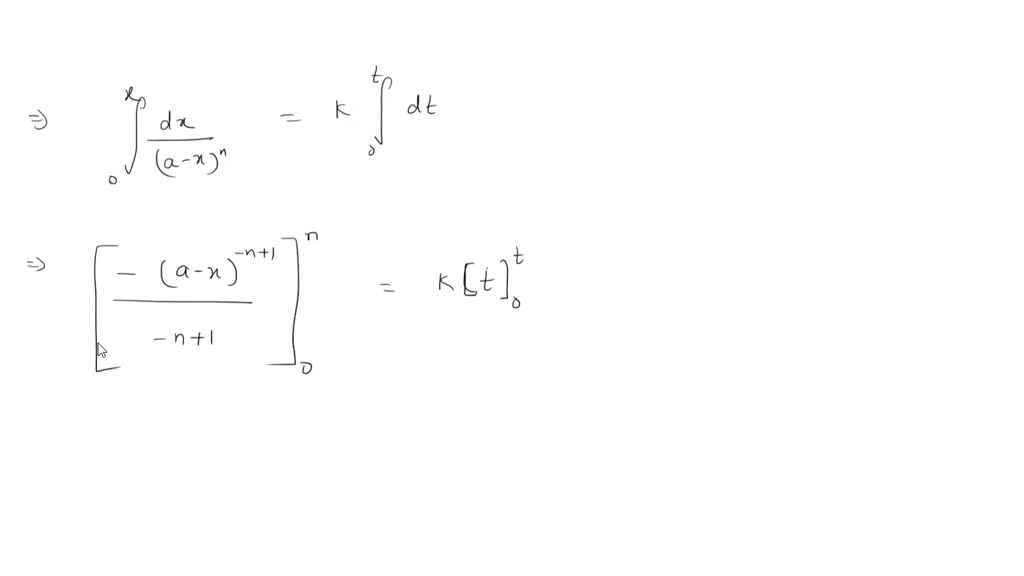
Solved Please Derive The Integrated Rate Law For The Nth Order N 2 Order Reactions As Well As The Half Life For An Order Greater Than 2 A P Keep The Derived Formula In Terms

How To Calculate Half Life For Zero Order Reactions Youtube
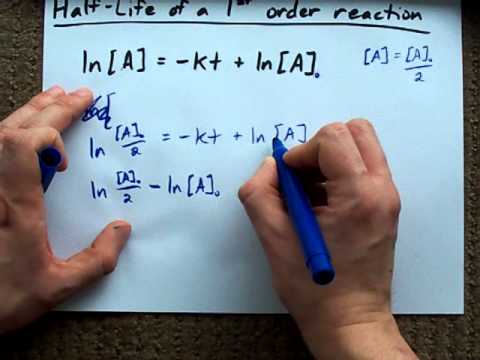
Half Life Of A First Order Reaction Derivation Youtube

Calculate The Half Life Of A First Order Reaction From Their Rate Constants Given Below A 200 S 1 B 2 Min 1 C 4 Year 1
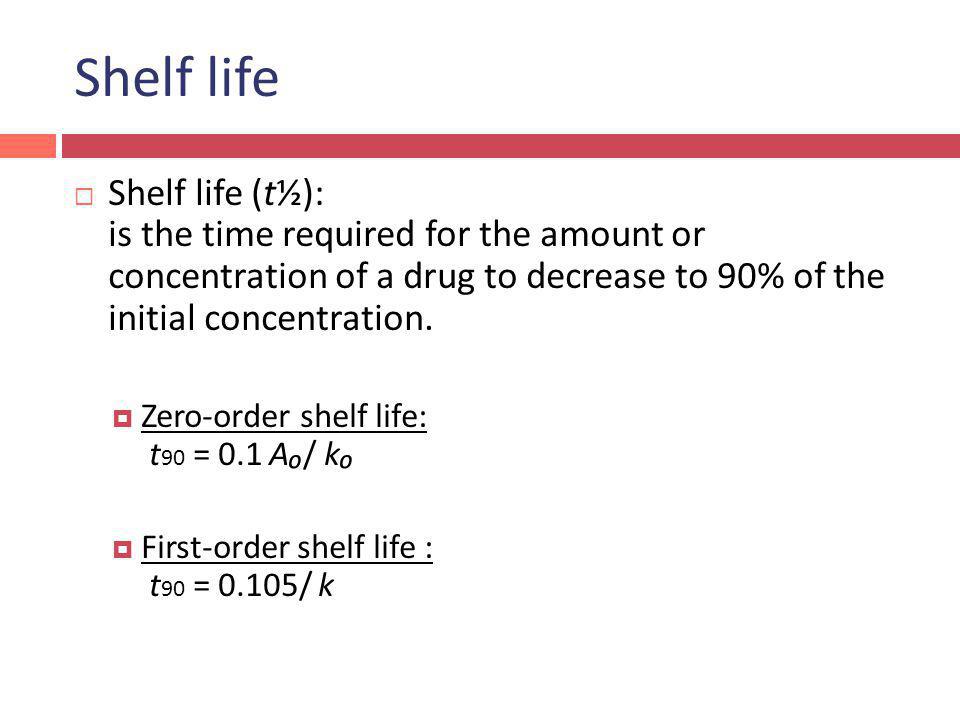
Kinetics Order Of Reactions Ppt Video Online Download
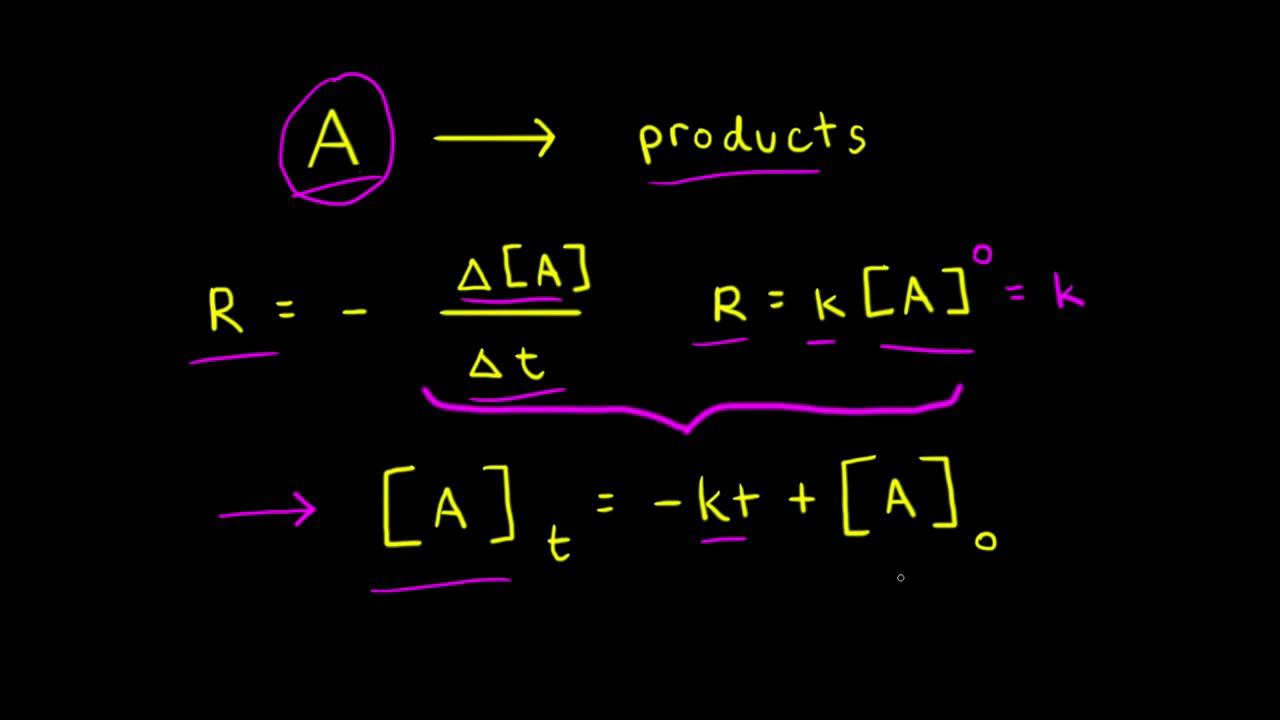
Zero Order Reactions Video Kinetics Khan Academy

Half Life Expressions Chemistnate

First Order Reaction Overview Equation What Is Rate Law Equation Video Lesson Transcript Study Com